生体情報・治療システム研究部門:Division of Bioinformation and Therapeutic Systems
研究上の強み:Research Advantage
- Major Research Strengths and Themes:
- 1) Defense Regenerative Medicine Engineering
- 2) Brain Health Innovation
- Core Technologies:
Leveraging the diverse biological effects of light and lasers, we develop novel diagnostic and imaging methods, as well as therapeutic and biological control technologies. These core technologies provide a scientific foundation for addressing critical challenges in defense medicine. - International Collaboration:
With a particular emphasis on blast injury research, we actively collaborate with international partners, including the U.S. military, NATO, and related institutions in allied countries. Through these efforts, we promote research that contributes to global security and international defense medicine.
We conduct regenerative medicine research aimed at improving the treatment of combat-related injuries. Our work includes the development of transplantation and regenerative therapies using human three-dimensional skin tissue models, with a focus on foundational technologies for practical implementation, such as light-based cellular activation and tissue regeneration.
We pursue research to protect and enhance brain function in defense personnel. This includes elucidating the mechanisms of blast-induced traumatic brain injury using laser-induced shock wave models, developing medical countermeasures, and investigating light-based cellular activation approaches to promote recovery from stress and brain fatigue.
メンバー:Staff
| 教授
Professor |
川内聡子(医博・工博)
Satoko KAWAUCHI, Ph.D. |
| 講師 Lecturer |
角井泰之(工博)
Yasuyuki TSUNOI, Ph.D. |
| 助教 Research associate |
杉山夏緒里(人間生物学)
Kaori SUGIYAMA (Human Biology, Ph.D.) |
| 客員研究員 Visiting Research Fellow |
西館泉(東京農工大学)
Izumi NISHIDATE, Ph.D. (Tokyo University of Agriculture and Technology) |
| Ibolja Cernak(Thomas F. Frist, Jr. College of Medicine, Belmont University) | |
| 水足邦雄(東京女子医科大学附属足立医療センター 耳鼻咽喉科)
Kunio MIZUTARI, Ph.D. (Department of Otorhinolaryngology-Head and Neck Surgery, Tokyo Women’s Medical University) |
|
| 栗岡隆臣(北里大学 耳鼻咽喉科・頭頸部外科)
Takaomi KURIOKA, Ph.D. (Department of Otolaryngology, Kitasato University) |
研究目的・方針:Mission
We conduct research to develop advanced diagnostic and therapeutic technologies that enhance survival and quality of life for patients suffering from trauma and combat-related injuries during large-scale disasters, terrorist incidents, and other emergency situations.
To achieve these goals, we apply a broad range of concepts and methods from photonics, spanning basic science to clinical application.
Our work also focuses on developing technologies that ensure the safety and operational performance of Self-Defense Forces personnel working under extreme conditions, through close collaboration with the Ministry of Defense and allied institutions, including the U.S. military.
主要研究テーマ:Main Research Topics
- ⑴ Development of burn diagnosis and treatment technologies
- ① Development of a burn depth diagnosis method using photoacoustic imaging
- ② Development of three-dimensional skin cultivation technology for transplantation
- ⑵ Development of wound infection control and biological decontamination technologies • Treatment of wound infections based on photodynamic treatment
- ⑶ Research on Blast-Induced Traumatic Brain Injury using laser-induced shock waves With the increasing frequency of terrorist attacks and military assaults involving explosive devices, blast-induced traumatic brain injury (bTBI) has emerged as a major public health concern, particularly in the United States. A defining characteristic of bTBI is that patients are often diagnosed as having mild injury during the acute phase, yet later develop chronic conditions such as cognitive impairment, migraine, sleep disorders, and psychiatric symptoms including depression and anxiety. An association with post-traumatic stress disorder (PTSD) has also been suggested. These symptoms are thought to result from the effects of blast shock waves on the brain ; however, the pathophysiology and underlying mechanisms of bTBI remain poorly understood, and effective medical countermeasures have yet to be established.
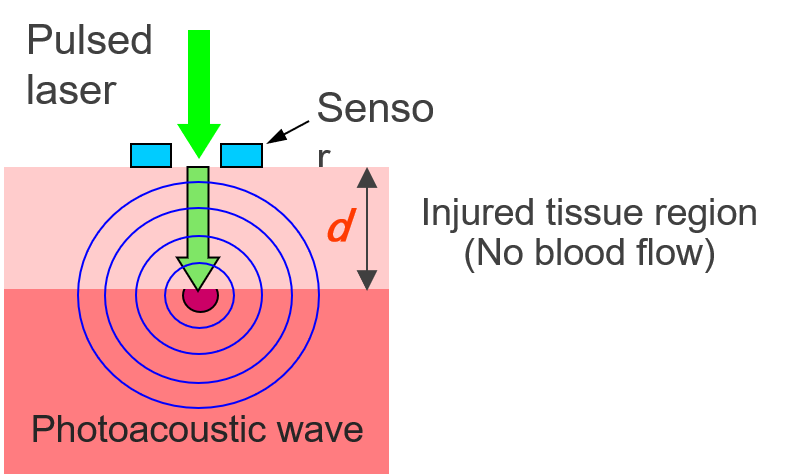
図1 光音響法による熱傷深度診断の原理
Figure 1 Principle of burn depth diagnosis using photoacoustic method
Burns are classified based on how deep the injury is. There are four types of burns: superficial burn, second-degree superficial dermal burn, deep dermal burn, and deep burn. It's very important to know exactly how severe the injury is so that the proper treatment can be chosen. This includes deciding if skin grafts are needed and what steps need to be taken to control infections. However, there are no established techniques for quantitatively measuring the burn depth. Currently, burn diagnosis relies on the visual observation by specialists. We therefore started to develop a new method to diagnose burn depth using photoacoustic imaging (S. Sato et al., J. Trauma, 2005). In burned tissue, blood flow is interrupted. Thus, when weak pulsed light at wavelengths that are easily absorbed by blood is irradiated onto the wound, the light can penetrate the injured tissue efficiently. On the other hand, the underlying uninjured tissue efficiently absorbs the light, generating photoacoustic waves (ultrasound) through adiabatic expansion. Photoacoustic waves can be detected at the tissue surface using acoustic sensors to calculate the depth of the injury This can be achieved by multiplying the propagation time of the acoustic wave by the known speed of sound (see Figure 1). We then conducted further research to improve the proposed method and created a working prototype system for clinical research in 2013. We are also researching ways to make the system more compact and cost-effective We have started sing light-emitting diodes (LEDs) as the light source for this method (Y. Tsunoi et al., Wound Rep. Reg., 2022).
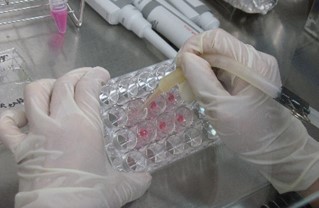
図2 三次元皮膚の培養
Figure 2 Three-dimensional skin culture

図3 三次元培養皮膚に対するPBMを行っている様子
Figure 3 PBM performed on three-dimensional cultured skin
One of these techniques is photobiomodulation (PBM; Figure 3). Iluuminating biological tissue with low-intensity light at certain wavelengths is known to activate the mitochondrial activity. This can increase the production of ATP (adenosine triphosphate) and the generation of reactive oxygen species (ROS). We started to apply this method to improve the viability of 3D skin during cultivation (Y. Tsunoi et al., Photochem Photobiol, 2022) , and we obtained a patent for a culture device based on this technology (Japan patent JP6956340 , 2021). Additionally, while three-dimensional skin culture is performed on porous membranes, there were challenges in detaching the cultured 3D skin from the membrane and in handling the detached soft skin when transplantation. We are developing a biodegradable porous membrane that allows transplantation of 3D cultured skin together with the membrane. We are using biodegradable polymers and microfabrication technology using ultrafast pulsed lasers, and we have confirmed that 3D skin can be successfully cultured and transplanted using this membrane (Y. Tsunoi et al., Tissue Eng.: Part A, 2023).
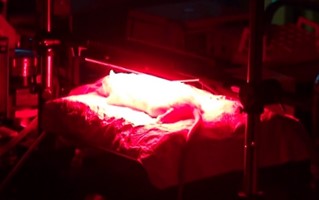
図4 ラット熱傷感染創部に対するPDT
Figure 4 PDT for infected burn wounds in rats
In the cases of severe cutaneous trauma, such as extensive deep burns, even when initial life-saving steps are successful, the mortality rate is still high because of infections that can lead to sepsis. This is especially fatal when the infection is caused by drug-resistant bacteria. Currently, there is no effective treatment for this. We are focusing on photodynamic treatment (PDT). PDT is a treatment that uses light to trigger a chemical reaction by exciting special drugs called photosensitizer. These drugs' excitation energy is then transferred to dissolved oxygen in tissue, generating singlet oxygen that can kill the surrounding cells. Due to this mechanism, PDT is expected to bet effective even against drug-resistant bacteria. We confirmed that PDT was effective in killing Pseudomonas aeruginosa and disrupting its biofilms, which protect the bacteria from drugs and immune cells, in vitro (R. R. Sarker et al., Photochem Photobiol, 2021). In addition, we applied PDT to prevent sepsis in a rat burn model. As a result, PDT greatly reduced the number of bacteria on the wound surface, prevented bacterial invasion into the body (blood and liver), and significantly improved the survival rate. Since PDT is a treatment method that is low-invasive, involving only illuminating the wound with light and applying the drug onto the tissue surface, it is easy to use in a clinical settings.

図5 ラット頭部へのLISW適用
Figure 5 LISW application to rat head

図6 ラット脳のマルチスペクトル
イメージング実験(S. Kawauchi et
al., J Biomed Opt, 2019)
Figure 6 Multispectral imaging experiment of rat brain (S. Kawauchi et al., J Biomed Opt, 2019)
Prior to our work, little was known about the real-time biological responses of the brain to shock wave exposure. By applying LISWs to the rat head and directly monitoring cerebral responses in real time (Fig. 6), we demonstrated that cortical spreading depolarization—a propagating wave of near-complete neuronal depolarization—occurs in the cerebral cortex, followed by prolonged hypoxic conditions (S. Sato et al., PLoS ONE, 2014).
We also found that the meninges located directly beneath the skull, which consist of the dura mater, arachnoid mater, and pia mater, are particularly vulnerable to shock wave–induced damage, with vascular injury in the dura mater being especially prominent (Fig. 7), likely due to acoustic impedance mismatch. As a consequence, we observed the accumulation of activated glial cells at injured sites, particularly at anatomical tissue boundaries, over days to weeks after injury, resulting in interface astroglial scarring (IAS) (S. Kawauchi et al., J Neurotrauma, 2024). Because interface astroglial scarring is a key pathological feature identified in postmortem brains of human bTBI patients, the successful reproduction of this pathology has enabled us to initiate therapeutic intervention studies using this experimental model.
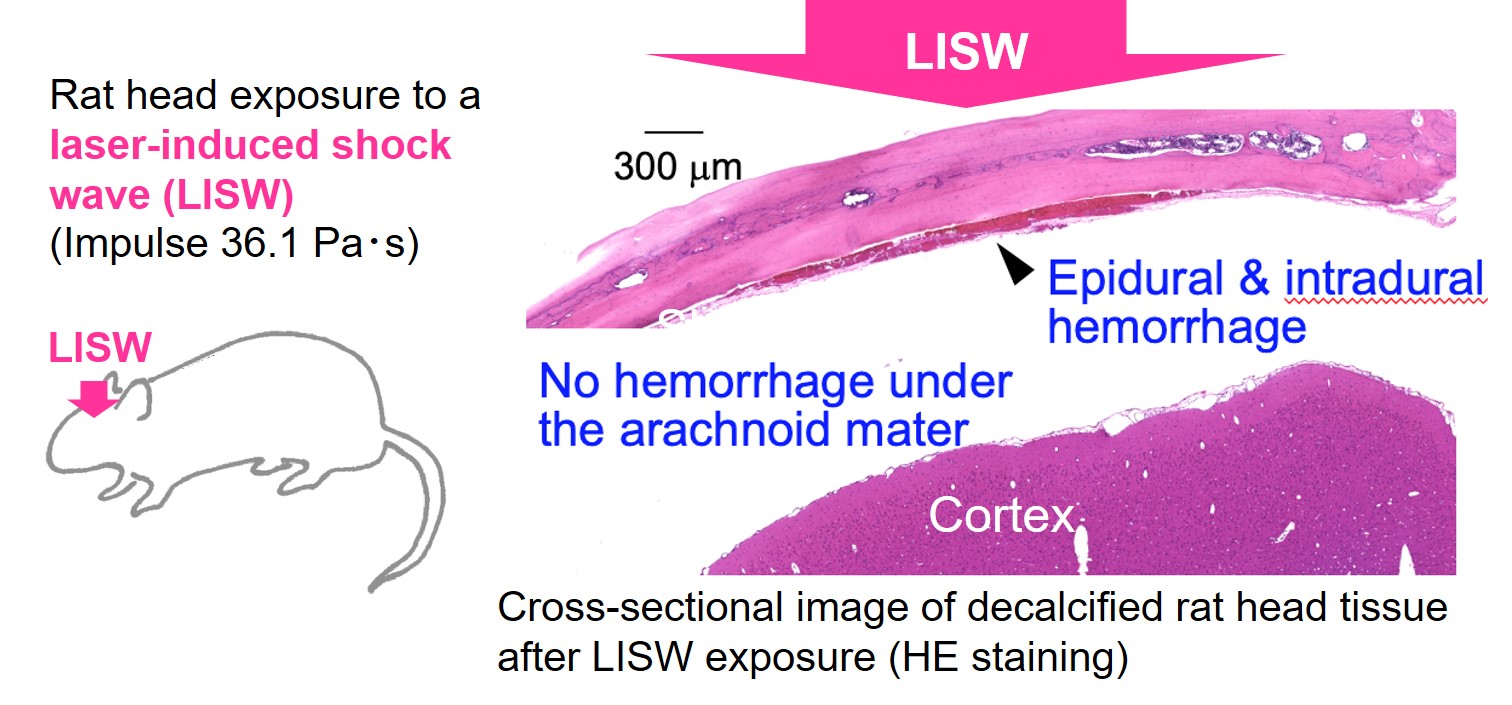
図7 LISWを適用したラットの髄膜損傷
Figure 7 Meningeal damage in the rat after LISW exposure
Our initial publication on LISW-based blast injury research (S. Sato et al., PLoS ONE, 2014) served as a catalyst for collaborative research efforts with the U.S. military. As part of this collaboration, the Japan–U.S. Blast Injury Forum was jointly launched in 2016. With growing international participation, the forum evolved into the International Forum on Blast Injury Countermeasures (IFBIC) at its fourth meeting in 2019 and has continued to expand. The 10th anniversary meeting, IFBIC 2026, is scheduled to be held in the United States in July 2026.
業績(令和5年度):Research Achievements (2023)
Article (in English)
Conference (in English)
Conference (in Japanese)







